Products
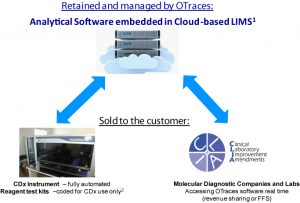
The OTraces technology platform drives multiple business opportunities. The software is embedded into a laboratory information management system (LIMS) wherein all patient and instrument data is stored in secure U.S. based servers. This software is the principal value-add for the company’s instrument systems now being developed for prostate and eventually breast cancer patient monitoring and screening, and access is now being offered to other companies through a unique cloud-based deployment program that could take higher predictive power to other companies and laboratories around the globe.
Cloud-Based Test Predictive Power
Enhancement Collaborations with Others
In partnership with Bio-IT Solutions, Inc. OTraces has developed a cloud-based system in which companies seeking higher accuracy send their raw biomarker data to OTraces’ servers for almost instantaneous processing, wherein the licensee or partner could achieve improved accuracy in exchange for paying OTraces either on a fee-for-service or revenue-sharing basis.
This is a highly interesting proposition because potential demand for this service is extensive, the business is highly scaleable, OTraces has strong potential pricing power as sole supplier, regulatory oversight in some cases should be limited, and associated processing costs for OTraces are nominal so profit margin potential should be very high.
Specific provisions of the cloud-based system include the following:
- On-demand service provision — The reach of the internet enables qualified partners to submit data and receive results wherever and whenever they want, without constraints of geography or time.
- Scaleability — Ability to run many concurrent analyses; easy to increase capacity as needed based on customer demand, complexity of algorithms or size of data sets to be analyzed.
- Product/Service Expansion — Straightforward to add new disease diagnoses, test methods, analytical techniques, data collection methods, reports and similar capabilities.
- Consistent Service Delivery — Software, algorithms, training sets, reports, and related capabilities delivered consistently and reliably with full traceability.
- Data Security and Access Control — The diagnostic platform will adopt the authentication, access control, data encryption and monitoring methods consistent with the best Internet applications that handle credit card information, patient medical records and similar private content.
CDx Instrument System
 This highly automated testing instrument is manufactured to company specifications by Hamilton Company, producing an accurate diagnosis without human interpretation. Hamilton has been as strong supporter of OTraces, having provided all customization, programming and support at no cost, so that OTraces could transfer the immunoassay methods to the Hamilton high speed ELISA robot.
This highly automated testing instrument is manufactured to company specifications by Hamilton Company, producing an accurate diagnosis without human interpretation. Hamilton has been as strong supporter of OTraces, having provided all customization, programming and support at no cost, so that OTraces could transfer the immunoassay methods to the Hamilton high speed ELISA robot.
The CDx is designed to optimize the performance and predictive power of the OTraces’ cancer screening reagent test kits. The system allows completion of one full run of all 5 immunoassays in the test panel in one shift, with no operator intervention after initial setup. In production configuration, the system will complete 80 cancers scores per day. When OTraces reaches full population screening level, production throughput will need to increase to at least 300 cancer scores per day. The Hamilton group of systems in this category is scalable so increasing this important parameter can be done when needed.
The OTraces technology measures blood protein biomarkers at unusually low levels of concentration, and uses biomarkers that have deep functional connections to immune response which OTraces uses as a virtual stethoscope on early detection and the various aspects of the disease process. The process of converting this data into highly accurate test results is performed remotely, using algorithms and software centrally stored in U.S. based servers by Bio-IT Solutions, Inc. exclusively and on OTraces’ behalf.
Reagent Test Kits
Maxim Biomedical operates a large in Rockville, MD and manufactures and supplies reagents and test kits used in the CDx instrument. Maxim GMP and ISO 13485 certified production capabilities and supplies the OEM manufacturing needs for some of the industry’s largest global companies.
TME Liquid Biopsy Dx Test Kit
TME Liquid Biopsy Dx immunoassay test for assessing progression of low Gleason Score prostate cancer to high Gleason Score aggressive prostate cancer, Active Surveillance.
PR Sera Dx Test Kit
PR Sera Dx Immunoassay test panel for detection of the presence of prostate cancer and discrimination of benign prostate hyperplasia (BPH) and aggressive from non aggressive prostate cancer.
BC Sera Dx Test Kit
BC Sera Dx Immunoassay test panel for detection of the presence of breast cancer in women.
In the development pipeline are an Ovarian, OV Sera Dx, and Lung, LG Sera Dx, cancer detection test kits.
Laboratory Information Management System
While Roche, Abbott, and others embed clinical chemistry software into the instruments in the field, OTraces uses a high-capacity Laboratory Information Management System to collect all patient data in a central location for the entire installed base. The LIMS offers several advantages:
- CDx Instruments will only run the company’s validated reagents.
- Bar coded reagent packaging allows the instruments and LIMS to connect all factory QC test results, available in real time as the tests are run in the field.
- All installed systems have a password-protected access to their own clinical site and confidentiality complies with HIPAA Standards and is fully encrypted.
- Factory QC results are compared to field test runs lot by reagent lot.
- Access to the software embedded in the LIMS is now being made available to others through a cloud-based capability operated by Bio-IT Solutions, Inc. wherein OTraces plans to participate either on a revenue-sharing or fee-for-service basis, with an expected growing list of companies across the globe.