Technology
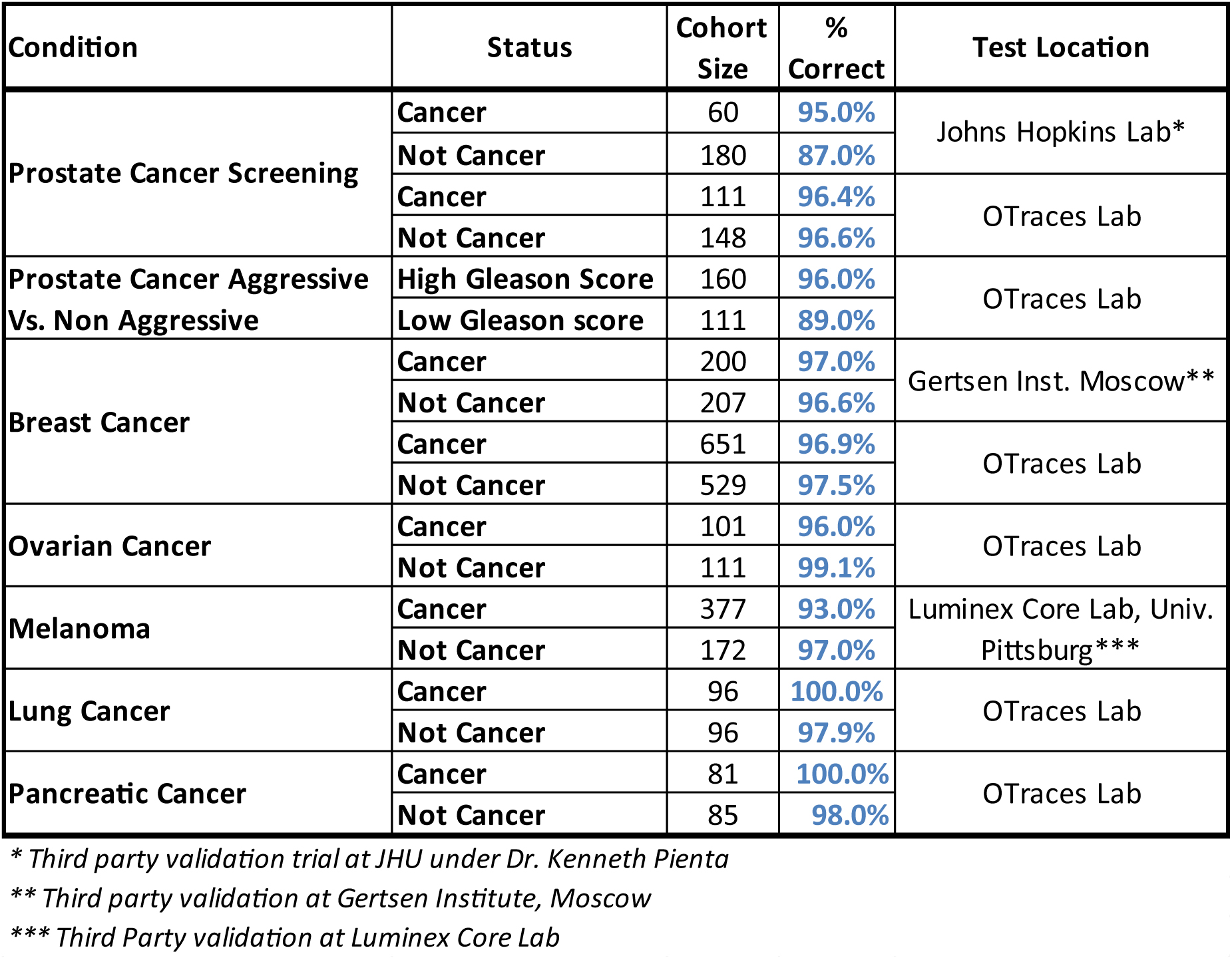
PREDICTIVE POWER TRACK RECORD
— in 6 cancer solid tumor types
— Consistent results almost 2,700 times
Compiled mostly over the past six years, this record includes third-party blinded results obtained at Johns Hopkins in prostate cancer, The Gertsen Institute in Moscow (breast cancer), and the Luminex Core Lab at the University of Pittsburgh for melanoma. All other tests were conducted internally at well-known regional hospitals
RESOLVING DIAGNOSTIC COMPLEXITY
A principal roadblock to achieving superior predictive power is presence of extraneous, random and sometimes random source of “noise” that corrupt conventional testing methods, and make it very difficult to model with any correlation method.
The following table illustrates some of the many distractions and diagnostic curve balls, in this case with non-cancer human Interleukin-6, as part of OTraces breast cancer screening panel conducted at the Gertsen Institute.
Non-Cancer Medical Conditions/Administered Drugs
Up-regulated by Interleukin 6
| Alcoholism | Cardiomyopathy | Rheumatoid Arthritis |
| Asthma | COPD | Schizophrenia |
| Autoimmune Disease | Major Depressive Disorder | Viral infections |
| Bacterial infections | Pulmonary Hypertension | Vaccines |
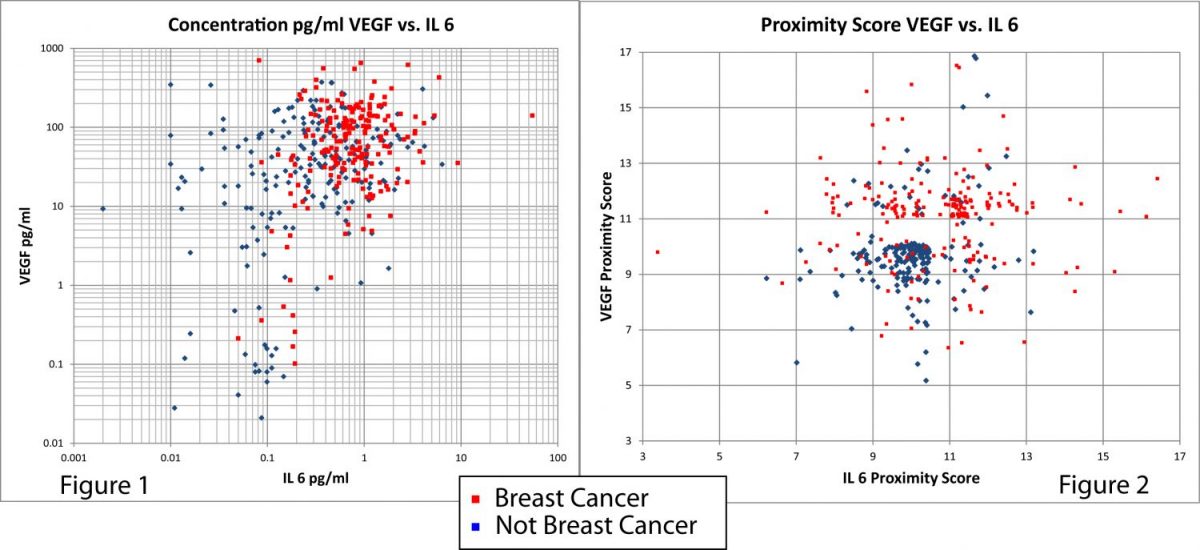
The start of the OTraces solution is see below in the transition from Figure 1 and 2.– where significant separation between breast cancer and non-cancer cells is achieved using only two biomarkers out of a typical 5 biomarker OTraces panel. This starts to shows the power of the company’s math – and – physics-based methodology.
EARLY DETECTION
Figure 3 traces biomarker levels in the Gertsen Institute validation trials for breast cancer, tracking them from healthy (-1 on the horizontal axis) through Stage 3, showing two noteworthy developments: (i) how quickly immune response takes place at early stage tumor formation; and (ii) how dramatically biomarkers surge during transition from healthy to Stage 0 tumors and most reach their highest levels at this stage —- further evidence of the accuracy enhancement potential of the OTraces technology: (iii) That this modality comprises a dynamic “motion picture” of tumor progression that is unique in cancer blood testing and an important advance in “liquid biopsy” that surpasses the known capabilities of DNA methods which are a virtual snapshot by comparison.
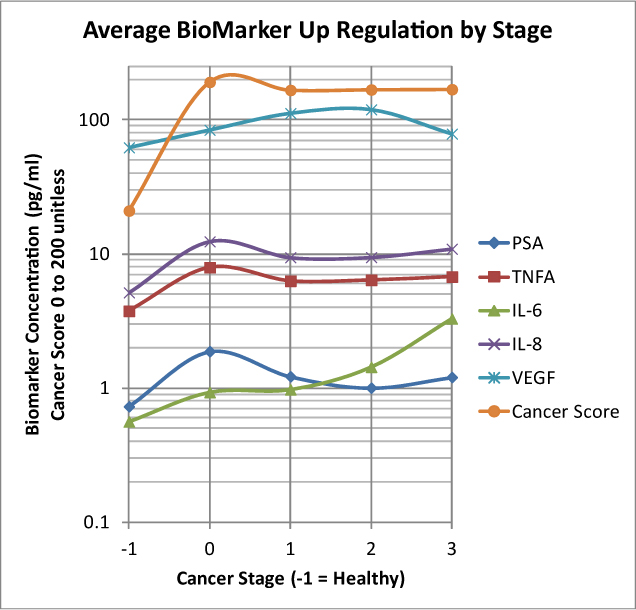
Figure 3, Action of Biomarkers by breast cancer stage
PROSTATE CANCER
Saving Costs by Reducing Unnecessary Biopsies and Saving Lives by Detecting Tumor Progression Before It is Too Late.
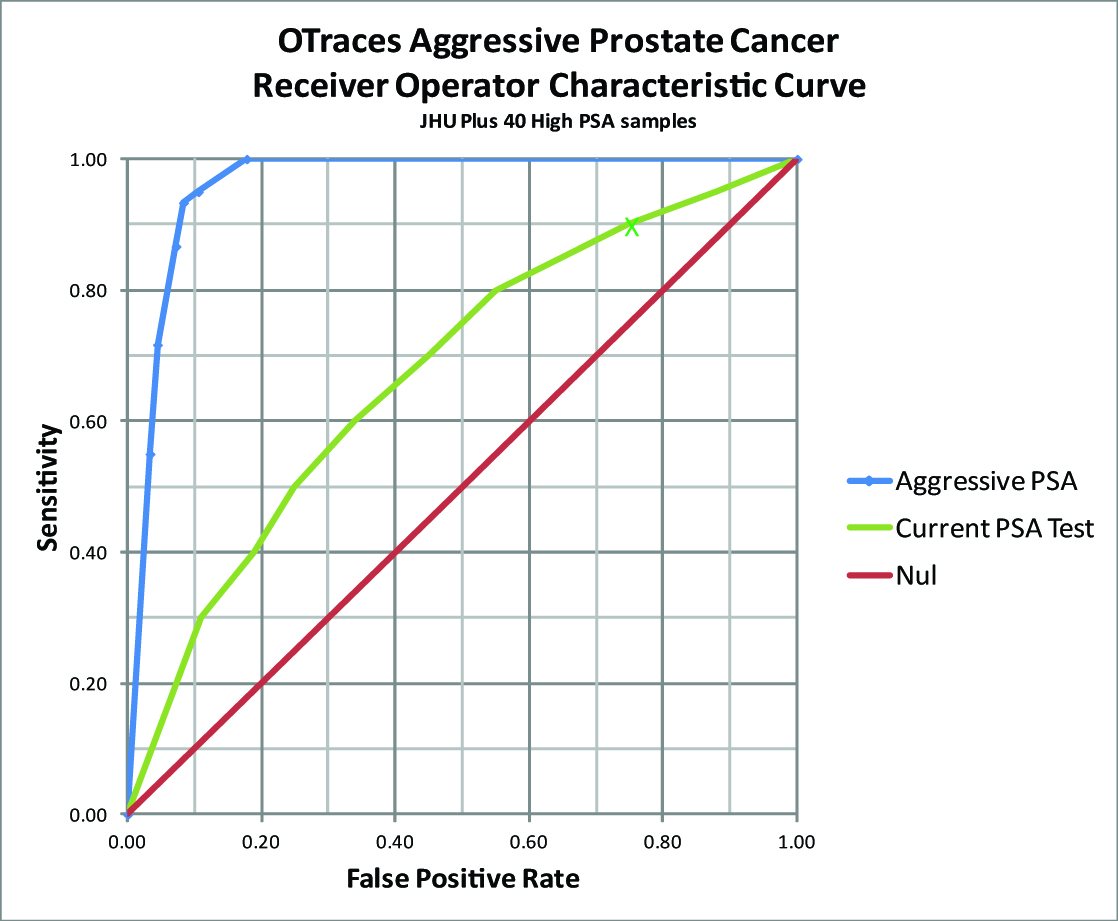
OTraces has completed one of three phases of third party blind validation of its prostate cancer test at Johns Hopkins Medical Center for the detection of aggressive PCa — an important unmet need in active surveillance patient monitoring as current A-S methods such as PSA often miss the progression from moderate to aggressive tumors until it is often too late to save the patient. Figure 4 below shows the resulting receiver operator curve (ROC) for the PSA test.
At a PSA level of 4 nano-gram/milliliter or higher in a man’s serum, the test achieves a false negative rate of 10%, 90% sensitivity, but gives up a 75% false positive rate (25% specificity). This means that the test misses 10 out 100 men with prostate cancer and 75% of those who measure positive for the PSA test get biopsies, though they do not have cancer.
In contrast OTraces test will only miss 5% of men with cancer. The false positive rate the test is only 11%, a far better outcome for men.